See the complete NECO Chemistry Questions and Answers for the 2025 June/July examination.
Are you a registered candidate for the 2025 NECO SSCE in any secondary school? If yes, you have to take all the information that I am going to be sharing in this article very seriously. Before you continue reading, I would like to let you know that particular article is specially for science candidates who will be writing chemistry in the examination.
NECO Chemistry Questions and Answers 2025 is one of the best tools you can use as you are preparing for the examination and guarantee yourself an excellent result in the subject thereafter. Take your time to carefully read this post so that you will have access to the likely examination questions and their answers before the date and time of the examination.
NECO Chemistry Questions and Answers 2025
Here is a detailed NECO chemistry answers for the 2025 examination:
CHEMISTRY OBJ
1-10: BBDDADEBCA
11-20: BDEABDBCEE
21-30: DEEECCCCEC
31-40: ABDAADCAAB
41-50: EDEDAEADDD
51-60: DBEBABBADA
Theory
(1ai)
(i) graphite
(ii) diamond
(1aii)
(i) animal charcoal
(ii) carbon black
(1aiii)
(i)The property of elements are a periodic function of their atomic number
(ii)Elements are arranged in the periodic table according to the order of increasing in their atomic weight.
(1bi)
Periodicity can be defined as the trend or recurring variation in element properties with increasing atomic number.
(1bii)
using mole = no. of atoms/avogadro’s constant
0.5 = No. of atoms/6.023 × 10²³
no. of atoms = 0.5×6.02 × 10²³ = 3.012 × 10²³ atom
(1ci)
Faraday’s first law of electrolysis state that the chemical deposition due to the flow of current through an electrolyte is directly proportional to the quantity of electricity (coulombs) passed through it.
(1cii)
2O²^- + 9^e —>2O²
no. of electron = 4
Q=20300C
G.M.V = 22.4dm³.
F = 96500C
Mole = Q/n,f
Mole = 20300/4 × 96500
Mole = 20300/386000
Mole = 0.05mol
Recall; = vol/G.M.V
0.05 = vol/22.4
vol = 0.05 × 22.4
vol = 1.12dm³
(1di)
Using H²SO⁴
H+ SO⁴^-¹
H+ OH^-
A+ Anode
OH —-> OH + e^-
2OH+(aq)+2OH(aq)—->2H²O(s)+O²(aq)
(1dii)
Tabulate
-Electrolyte- (I)teraoxosulphate(iv)acid
(ii)Ester
-non electrolyte-
(iii) phenol
(1ei)
(i) mercury
(1eii)
(i) No. of electron in Y = 16
(ii) No. of mass number = 16 + 18 = 34
(iii) sulphur
======
(2ai)
The basicity of an acid is the number of replaceable hydrogen ions N+ in one molecule of the acid.
(2aii)
(i) H3p04 —> basicity = 3
(ii) HC00H —> basicity = 1
(iii) H2S04 —> basicity = 2
(2bi)
-Temperature of the reacting system
-Pressure of the reacting system
-Concentration of the reacting system
(2bii)
-Nature of reactants
-Concentration/Pressure of reactants
-Surface area of reactants
(2ci)
In a tabular form
Under S/N
I, II
Under Indicator:
Methyl orange, Phenolphthalein
Under colouric acid:
Pink, Colourless
Under colour of endpoint:
Orange, colourless
Under colour in base:
Yellow, pink
Under suitable for:
Strong acid and weak base,
Weak acid and strong base
(2cii)
I. Nitrogen
1S, 2S, <-2P->
↑↓ ↑↓ ↑↑↑
III. Fluorine
1S, 2S, <-2P->
↑↓ ↑↓ ↑↓↑↓↑
II. Aluminium
1S, 2S, 2P, 3S, 3P
↑↓ ↑↓ ↑↓↑↓↑↓ ↑↓ ↑
(2di)
(i) Heat is evolved liberating hydrogen gas
(ii) Decolourization of purple KMn04 with deposit of sulphur
(iii) Black residue of carbon due to dehydration
(2dii)
(i) Glucose is used as an immediate source of energy for sick people and sportsmen
(ii) It is used in the manufacture of sweets.
(3)
(3ai)
Cl2(g) + 2NaOH(aq) + Nacl + H2O(l)
[Cold dilute]
3Cl2(aq) + 6NaOH(aq) + 5Nacl(aq) + 3H2O(l)
[Hot Conc.]
(3aii)
Green to Brown
(3aiii)
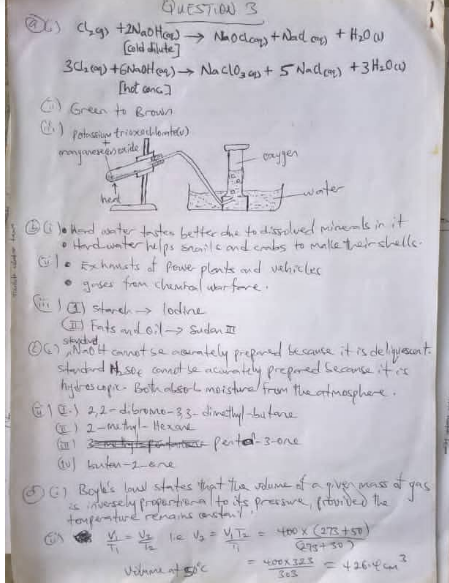
DIAGRAM
(3bi)
(i)Hard water tastes better due to dissolved minerals in it
(ii)Hard water helps snails and crabs to make their shells
(3bii)
(i)Exhaust of power plants and vehicles
(ii)Gases from chemical warfare
(3biii)
(i)Starch —> Iodine
Fats and oil —> Sudan III
(3ci)
(i)Standard NaOH cannot be accurately prepared because it is deliquescent
(ii)Standard H2SO4 cannot be accurately prepared because it is hydroscopic. Both absorb moisture from the atmosphere.
(3cii)
(i)2,2-dibromo-3,3dimethyl-butane
(ii)2-methyl-Hexane
(iii)Pentan-3-one
(iv)Butan-2-one
(3d)
(i)Boyl’s law states that the volume of a given mass of gas is inversely proportional to its pressure, provided the temperature remains constant.
(ii)V1/T1 = V2/T2 i.e V2 = V1T2/T1
= 400 x (273+150)/(273+30)
Volume at 50°c = 400×323/303 = 426.4cm^3
(4)
(4ai)
(I) Water is necessary for corrosion to take place whereas burning is different in the presence of water.
(II) Boiling takes place at a specific temperature called boiling point whereas evaporation takes place at all temperatures.
(4bi)
A concentrated solution is one containing large amount of the solute dissolved in water.
(4bii)
(i) The positions of the ions in the electrochemical series.
(ii) The concentration of the ions in the electrolyte.
(iii) The nature of the electrode.
(4ci)
R.M.M = 2[Al] + 3[(S) + 4(O)]
= 2(27) + 3[32 + 4(16)]
= 54 + 3[96]
=342
(4cii)
Aluminium tetraoxosulphate(vi).
(4ciii)
Propane -1,2,3 -triol and salt.
(4di)
(i) Soapless detergents do not form scum with hard water, soaps do.
(ii) Soaps are biodegradable; certain soapless detergents are not and hence cause pollution of water ways.
(4dii)
OH and COOH
(4diii)
v1p1=v2p2 ie v2 = v1p1/p2
= 300×760/800 = 285cm³
(4div)
Change = +13 – 10 = +3
(4dv)
Aluminium ion

See also:
NECO Civic Education Questions and Answers for 2025 | Essay and Objectives
NECO Biology Questions and Answers for 2025 | Essay and Objectives
NECO Government Questions and Answers for 2025 | Essay and Objectives
NECO Biology Practical Specimen, Questions And Answers 2025 | Complete Specimen And Solutions
NECO Home Management Questions And Answers 2025 | Essay And Objectives
NECO Mathematics Questions And Answers For 2025 | Theories And Objectives
NECO Agric Questions And Answers 2025 | Essay And Objectives
NECO Marketing Questions And Answers 2025 | Theories & Objectives
How to Score an “A” in NECO Chemistry Examinations
I have discovered that a lot of candidates do find it difficult to answer NECO chemistry examination questions, thereby contributing to their general poor performance in the examination every year.
That is the reason why I have decided to include this vital section in the article you have been reading. For you to perform excellently in the NECO chemistry examination, there are certain things that you must do.
In this section of the article, I will be showing you some tips that are very essential for you to score an “A” in chemistry at the end of the examination. Those things that you should include the following:
1. Cover your Syllabus
For you to make an “A” in the NECO Chemistry examination, it is a prerequisite that you must finish the NECO syllabus on chemistry. This will enable you to have good courage as you go ahead to write your examinations. This, in turn, will guarantee your good success at the end of your examination.
2. Follow Instruction
Once you get into the examination venue and you have gotten your question paper and answer booklets, you have to read the given instructions carefully before you can start answering the questions.
After reading the instruction, ensure that you follow every given instruction strictly to the end to avoid being penalised for lack of adherence to instructions.
3. Complete your questions
Before you submit your answer booklets, ensure that you have answered completely and accurately all the required questions. For example, if you are required to answer five questions, make sure that you do not answer less than or above that number.
Failure to adhere to this rule will attract a punishment that might lead to your poor performance at the end of your examination. To add to that, do not submit your booklet without properly reviewing your answers.
Get more tips: How to Pass your NECO Examination at One Sitting
I believe you have found this article helpful. In case of any other question about NECO Chemistry Questions and Answers 2025, kindly reach out to us through the comment section below this article.
