Join one of the lucky candidates who will have access to the May/June WAEC Chemistry Questions and Answers for 2025 | Objectives and Theories. It is not everyone who is writing the 2025 WAEC examination will be privileged to see the information that I am going to reveal to you here.
Are you a candidate who is sitting for the May/June WAEC examination for 2025? This article is very important for you especially if you are a science student.
In this article, I am going to be revealing to you those questions that you are going to see in chemistry on the examination date. If you are interested in becoming one of the early birds who will have access to the exact questions and the answers, kindly read this article to the end.
First of all, let me start by answering some of the frequently asked questions about WAEC chemistry questions every year. These questions are going to give you more guides about the 2025 WAEC questions and how you would be able to answer them.
- How Many Questions are in WAEC Chemistry
- How to Answer WAEC Chemistry Questions
- Reasons for Poor Performance in Chemistry
- What You Should Read for WAEC Chemistry 2025
- WAEC Chemistry Objective Questions for 2025
- WAEC Chemistry Objective Answers for 2025
- WAEC Chemistry Essay Questions for 2025
- WAEC Chemistry Theory Answers for 2025
How Many Questions are in WAEC Chemistry
The WAEC chemistry examination is usually made up of not less than three subsections with each subsection containing a specified number of questions.
This section presents to you the number of questions that you are going to see in the WAEC Chemistry Examination for 2025.
Paper 1
This part is usually the objective part of the Chemistry examination. It is usually made up of 50 questions in all and candidates are expected to answer all the questions contained therein.
These are usually compulsory questions that you are expected to finish. They carry 1 mark respectively, making a total of 50 marks.
Paper 2
In this section, you will be given about 7 questions and would be required to answer just 5 questions in all. While entering this part, it is necessary to check for the question(s) that have been made compulsory by WAEC.
Paper 3
The paper 3 is made up of 3 questions which are usually practical questions. You would be expected to answer all the questions compulsorily.
How to Answer WAEC Chemistry Questions
They are quite good number of students who have concluded that chemistry is one of the hardest subjects to write in WAEC examination. This could be as a result of how they were thought in their respective secondary schools. Some of them do not even have a good knowledge of chemistry.
For one to pass chemistry examination at one sitting in WAEC, they are some principles that must be put in practice. These are the rules that are applicable during the time of writing the examination.
If you follow the guidelines that I am going to give you in this section, then you will make an excellent result in chemistry
The principles for answering WAEC chemistry examination include the following:
- Read Instructions
Once you have settled to take your examination in the examination hall, the next thing that you must do first is to read through the instructions on top of the page before you can proceed with reading the questions.
Most times, some instructions are specific to some questions, maybe one or two questions. Here, you still have to take those instructions very serious.
2. Read Carefully
Before you can proceed to answering WAEC chemistry questions, it is highly recommended that you read carefully to understand any question before you can start to answer the questions.
It is possible to see questions that would be similar to what you have been seeing before, probably in past questions, but they are not the same.
This is the point where it is very necessary that you meticulously go through the question before you answer, to avoid choosing the wrong option for objectives and giving wrong explanations for theory questions.
3. Take Note of Compulsory Questions
In every WAEC examination, there is/are usually some questions that are specially made compulsory by the West Africa Examination Council. These questions are always seen in the paper 2 which is theory aspect of it.
You must ensure that you are able to answer those compulsory questions before you can think of answering any other ones. The compulsory questions do carry special mark.
4. Start with the Simplest
The complexity of every question in WAEC chemistry varies. After you have gone through the questions, you would be able to tell which of the questions are simple and the ones that are difficult.
The best approach in such case requires that you answer the questions from the simplest to the most complex ones. This is important because it will help to cushion you against examination tension.
Not only that, it also helps in the management of your time. If you are finding any question difficult to answer, you have to leave it and go to the next. Thereafter, you can re-visit those questions that you have left unanswered.
5. Attempt all the Question
Though it is advisable that you start answering your question from the simplest, leaving the more difficult ones at the first attempts, it does not imply that you should submit you examination without answering those ones you skipped.
It quite understandable that you may not know all the asked questions, you are still required choose your answers even the ones that you do not really know very well.
Sometime, your guess can fall in place and become the right answer. So always ensure that you attempt all you question before you submit.
6. Review your Answers
After you have attempted the entire given questions, you still have to go through all the answers to check for any errors and possible corrections.
By going through the questions, you would be able to see any skipped question(s), if any and the ones you mistakenly clicked the wrong options.
Reasons for Poor Performance in Chemistry
It is an error to assume that WAEC chemistry examination is hard. Failure to perform excellently in WAEC chemistry examination is a question of whether the candidate knows the right things to do and if he/she is doing it right.
The poor performance that have been seen in some candidates’ results over the years are as a result of poor application of the basic principles for writing WAEC chemistry examination and some other factors that I am going to show you here.
1. Inadequate Preparation
This is the most important aspect of the reasons for poor performance in WAEC Chemistry that should be considered.
It is obtainable in all areas that when there is poor preparation for any examination they will be poor result. As a good student, you are required to prepare for the WAEC chemistry examination to the point that you would be sure of scoring high.
2. Poor Time Management
Time management is another important factor that can lead to poor performance in every examination. You should learn how to manage your time especially when you are in the examination hall.
The practice of time management starts from the period of the candidate’s preparation for the examination. Once you can answer your past questions comfortably with the limited time that will be provided, you will still manage your time very well on the examination date.
3. Lack of Adherence to Given Instructions
There are some students who are fond of ignoring instructions when come for examination. Jumping into answering of questions without reading the given instructions is very wrong. This contributes in a big way to poor performance in the examination.
If you want to have good performance in WAEC chemistry, you have to strictly pay close attention to any given instructions.
4. Submitting Incomplete Answers
When a candidate submits the examination without attempting all the questions, it will automatically affect his/her score. It is important you answer all the questions before your submission.
What You Should Read for WAEC Chemistry 2025
In WAEC chemistry examination, they are some topics that are considered very important. These topics are where most of the questions that are repeatedly asked by the WAEC for every year.
As you continue to read this section, I am going to reveal those topics to you. If you are able to read the chemistry topics that I am going to show you here very well before the examination, it means that you can attempt up to 90% of WAEC chemistry questions correctly.
These topics include the following:
- Nature of Matter
- Separation Techniques
- Atomic Structure
- Chemical Combination
- Kinetic Theory of Matter
- Gas Laws
- Acids, Bases and Salts
- Periodic Table
- Non-metals and their Compounds
- Metals and their Compounds
- Electrolysis
- Energy and Chemical Reactions
- Chemical Equilibrium
- Rate of Reaction
- Air and Air Pollution
- Water, Solution and Solubility
- Shapes of Molecules and Solids
- Radioactivity
- Hydrocarbon
- Organic chemistry
However, you should note that reading with the WAEC syllables is the best practice. The topics listed above are just suggestion of areas that you can concentrate so as to get a good result in the WAEC chemistry examination.
WAEC Chemistry Objective Questions for 2025
The following are what you are likely going to see in the 2025 WAEC chemistry examination for objective section.
1. A mixture of sugar and sulphur can be separated by
A. dissolution in water, evaporation and filtration
B. filtration, evaporation and dissolution in water
C. dissolution in water, filtration and evaporation
D evaporation dissolution in water and filtration
2. Which of the following is a physical change?
A. Freezing ice-cream
B. Dissolving calcium in water
C. Burning kerosene
D. Exposing white phosphorus to air.
3. The percentage of water of crystallization in ZnSO.7H2O is
A. 33%
B. 44%
C. 55%
D. 87%
[Zn = 65, S = 32, O = 16 , H=1]
4. 0.0075 mole of calcium trioxocarbonate(IV) is added to 0.015 mole of a solution of hydrochloric acid. The volume of gas evolved at s.t p. is
A. 224cm3
B. 168cm3
C. 112cm3
D. 100cm3
[Molar volume of a gas at s t.p = 22.4dm3]
5. A gas exerts pressure on its container because
A. the molecules of a gas collide with the walls of the container.
B some of the molecules are moving faster than others.
C. of the collisions of the molecules with each other
D. of the mass of the molecules of the gas
6. The basic assumption in the kinetic theory of gases that the collisions of the gaseous molecules are perfectly elastic implies that the
A. forces of attraction and repulsion are in equilibrium
B. gaseous molecules can occupy any available space
C. gaseous molecules will continue their motion indefinitely
D gases can be compressed
7. If an atom is represented as 1123X, which of the following deductions is correct?
A. It contains 12 protons.
B. It forms a covalent chloride.
C. Its atomic number is 23
D. It is an alkali metal.
8. If the relative molecular mass of an element is not a whole number, it can be deduced that the element is
A naturally radioactive
B. abundant in nature
C. a transition metal
D. an isotopic mixture
9. Cathode rays cause an object placed behind a perforated anode to cast a shadow on the screen. This observation shows that the rays
A. are positively charged
B. are negatively charged
C. have mass
D. travel in straight lines
10. Which quantum number divides shells into orbitals?
A. Principal
B. Azimuthal
C. Magnetic
D. Spin
11. The type of bonding in [Cu(NH3)4]2+ is
A. coordinate B. electrovalent C. metallic D. covalent.
12. The mixture of gases used in a photographer’s flash tube is
A. Argon and krypton
B. Krypton and Xenon
C helium and argon
D. argon and xenon
13. When sodium trioxocarbonate(IV) decahydrate loses its water of crystallization to the atmosphere, the process is
A deliquescence
B. efflorescence
C. hygroscopic
D. effervescence
14. Water can be obtained as the only product during the
A. combustion of hydrocarbons
B. neutralization of an acid by a base
C. combustion of hydrogen
D. Electrolysis of brine.
15. If 10.5g of lead (II) trioxonitrate (V) is dissolved in 20cm³ of distilled water at 18°C, the solubility of the solute in moldm-3 is
A. 1.60
B. 5.25
C. 16.00
D. 25.00
[Pb = 207 N = 14, O = 16]
16. For a given solute, the concentrations of its saturated solution in different solvents are
A. the same at the same temperature
B. different at the same temperature
C. the same at different temperatures
D. constant
17. The major source of oxides of nitrogen is from the burning of
A. coal
B. wood
C. fuel
D. chlorofluorocarbons
18. The acid used in electrolysis of water is dilute
A. HNO3
B. CH₂COOH
C. H2SO4
D. HCI
19. What volume of 1.5 M solution of KOH would contain 0 045 moles? A 67.50cm³
B 30.00cm3
C. 6.75cm3
D 3.00cm3
20. The salt formed from a reaction between citric acid and sodium hydroxide in solution will be
A. acidic
B. basic
C. complex
D. neutral
21. The colour change observed when testing for reducing agents using acidified potassium heptaoxodichromate(VI) solution is
A. yellow to purple
B. orange to green
C. green to orange
D. purple to yellow
22. The oxidation state of Cr in K2Cr2O7 is
A. +7
B. +6
C. +5
D. +4
23. Which of the following metals is purified commercially by electrolysis?
A. Zn
B. Fe
C. Sn
D. Cu
24. What current will deposit 3.25g of zinc in 2 hrs?
A 3.25A
B. 2.00A
C.1.34A
D 0.67A
See: Updated May/June WAEC Timetable for the 2025
[Zn = 65, F = 96500C mol–¹]
25. C(s)+H2O(q) → H2(q)+CO(q) ∆G for the reaction above at 1300K is – 43kJ At this temperature, the reaction is
A not feasible
B. at equilibrium
C. feasible
D. exothermic.
26. Two equal bulbs, one containing ammonia and the other nitrogen, are opened mouth-to-mouth to each other at room temperature. The entropy in the mixture of gases is likely to
A. remain unchanged
B increase
C. decrease
D. change.

27. In the diagram above, which of the curves represents the evolution of oxygen with time in the equation 2KCIO3(s) → 2KCl(s) + 3O2(g)?
A. X
B. Y
C. Z
D. R
28. A catalyst increases the rate of a chemical reaction by providing a path that
A. raises the activation energy
B. increases the temperature
C. lowers the activation energy
D. increases the concentration.
29. CuO(s) + H2(g) ↔ Cu(s) + H2O(l).
What is the effect of increasing the pressure on the equilibrium reaction above?
A. The equilibrium is shifted to the left.
B. The equilibrium is shifted to the right.
C. There is no effect.
D. More H2(g) is produced.
30. The equilibrium of an endothermic reaction which proceeds with an increase in volume can be shifted in the reverse direction by
A. increasing the temperature and decreasing the pressure
B. increasing the pressure and decreasing the temperature
C. decreasing the temperature and increasing the pressure
D. decreasing the pressure and decreasing the temperature
31. The oxidation of ammonia in excess air produces
A. NO
B. N2O
C. NO₂
D. N₂O4
32. Hydrogen sulphide gas can act as
A. an oxidizing agent
B. a dehydrating agent
C. a bleaching agent
D. a precipitating agent
33. The gasification of coke is used for the manufacture of
A. producer gas
B. natural gas
C. synthetic gas
D. industrial gas
34. The gas that is most useful in protecting humans against solar radiations is
A. chlorine
B. ozone
C. carbon (IV) oxide
D. hydrogen sulphide.
35. Which of the allotrope of carbon is a constituent of a lead pencil?
A. Graphite
B. Diamond
C. Lampblack
D. Soot.
36. Which of the following metals will show the highest metallic character?
A. Zinc
B. copper
C. Barium
D. Calcium
37. Copper metal dissolves in concentrated trioxonitrate(V) acid with the resultant evolution of
A. CO2
B. SO2
C. NO2
D. CO
38. The type of iron that is best suited for welding, making nails, chains and iron rods is
A. pig iron
B. wrought iron
C. cast iron
D. iron pyrites.
39. In the electrolytic extraction of aluminium, the function of the molten cryolite is to
A. precipitate aluminium hydroxide
B. lower the melting point of aluminium oxide
C. act as a raw material
D. act as a solvent
40. One of the products of the thermal decomposition of sodium trioxonitrate(V) is
A. sodium
B. nitrogen
C. sodium dioxonitrate (III)
D. sodium oxide.
41. A cracking process in petroleum refining can be represented by
A. heptane to heptene
B. heptane to 3-methylhexame
C. heptane to propene and butane
D. heptane to 2,3,3-trimethybutane.
42. Which of the following molecule has two other positional isomers?
A. CH3CH₂Br
B. CH3CHBr
C. CH3CBr₂F2
D. CH₂CHBrCH₂Br
43. Which of the following class of compounds can exist as dipolar ions in solution?
A. Alkanoic acids.
B. Fatty acids.
C. Dialkanoic acids.
D. Amino acids.
44 Lucas reagent is used to test for
A. alkanes
B. alkanoic acids.
C. alkanols
D. amines.
45. A compound commonly used for sterilization and preservation of specimens and food is
A. ethanol
B. benzene
C. ether
D. ammonia.
46. An organic compound reacted with bromine water to give a colourless solution. The compound is probably an
A. alkene
B. alkanal
C. alkyne
D. alkanone.
47. Which of the following hydrocarbons is mainly used as fuel?
A. Methylene.
B. Ethylene.
C. Methane
D. Ethyne.
48. The molecular formula of a common organic laboratory anaesthetic is
A. CHCl3
B. CHI3
C. CCl4
D. CHF3
49. The simplest branched-chain hydrocarbon is
A. ethane
B. ethene
C. propane
D. butane
50. Organic molecules that have the suffix-ene are unsaturated hydrocarbons that have
A. a single bond
B. a double bond
C. a triple bond
D. an ionic bond
See also: How to Check WAEC Result Online – 2025
WAEC Chemistry Objective Answers for 2025
- C
- A
3. A
ZnSO4.7H2O = 65 + 64 + 7(18) = 287
% water of crystallization = 126/287 x 100/1 = 44% (A)
4. CaCO3 + 2HCl → CaCl2 + CO2 + H2O
1 mole of CaCO3 produce 1 mole CO2
∴ 0.0075 mole CaCO3 will produce 0.0075 mole CO2
1 mole of CO2 occupy 22.4dm3 = 22400cm3
∴0.0075 CO2 will occupy 0.0075 x 22400cm3
= 168cm3
5. A 6. B 7. D 8. D 9. A 10. B 11. A 12. B 13. B 14. C
15. Pb(NO3)2 = 331
20 cm3 of distilled water is saturated by 10.5g Pb(NO3)2
1000cm3 will be saturated by
= 10.5/20 x 1000/1 = 525gdm-3
In moldmol-3 = 525/331 = 1.59 = 1.60moldm-3 (A)
16. A 17. C 18. C
19. 1.0M of 1000cm3 contains 56g of KOH
1.5M of 1000cm3 will contain 84g of KOH
∴1.5M contain 84/56 = 1.5M
i.e 1.5M = 1000cm3
∴0.045 moles 1000/1 x 0.045 = 30.00cm3 Ans = B
20. D 21. B
More answers loading…>>
See also: 2025 WAEC Biology Practical Specimen, Questions and Answers
WAEC Chemistry Essay Questions for 2025
These are the kinds of theory questions that you are likely going to see in WAEC Chemistry for 2025.
SECTION A
1. Compound A consisting of carbon and hydrogen only. The compound was found to contain 80% carbon by mass.
(a) Calculate the empirical formula of compound A using the data above.
(b) The relative molecular mass of compound A was found to be 30.
Use this information to deduce the molecular formula of compound A.
[H = 1.00 C = 12.00]
2. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
3. Calculate the percentage by mass of silicon tetrachloride. [2 marks] 4. Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5.
(a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
5. The first ionization energy of chlorine is +1260KJmol-1.
(a) Define the term first ionization energy.
(b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
6. An aqueous solution has a pH of 4.0.
(a) (i) What is the hydrogen ion concentration of the solution?
(ii) What effect will it have on litmus paper?
(iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq).
(b) (i) Differentiate between a fine chemical and a heavy chemical.
(ii) Name two sources of air pollution.
(iii) Suggest one way of reducing air pollution in cities
WAEC Chemistry Theory Answers for 2025
No. 1a
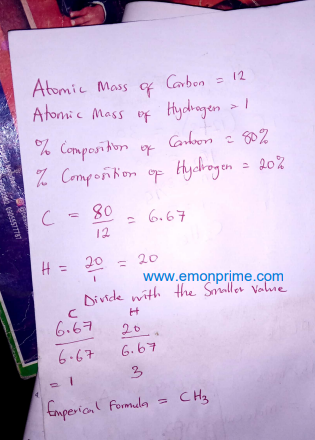
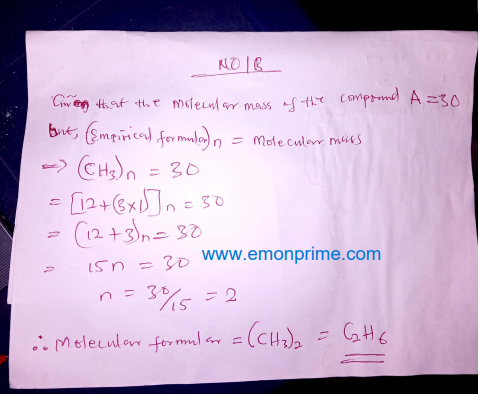
More answers loading…>>
Keep refreshing this page if you wish to get all solutions to the chemistry theory question for 2023.
Related Posts
WAEC English Questions And Answers 2025 | Objectives, Test Of Orals and Essay
WAEC Economics Questions And Answers 2025 | Theories And Objectives
WAEC French Questions And Answers 2025 | Theories And Objectives
WAEC Mathematics Questions And Answers 2025 | Theory And Objectives
WAEC Physics Practical For 2025 | Specimens/Apparatus, Questions And Answers
WAEC Chemistry Questions And Answers For 2025 | Objectives And Theories
WAEC Biology Practical Specimen 2025 | Questions And Answers
Complete WAEC Physics Questions And Answers For 2025 (Objectives & Theory)
I believe that you have found this article very useful. For any other questions about the WAEC Chemistry Questions and Answers for 2025, kindly make use of the comment section.
Please do well to share this information with others
