Here is a complete JAMB Chemistry Questions and Answers 2025 for Day 1, 2, 3 & 4
Have you seen the JAMB Chemistry Questions and Answers for 2025? If you have not seen them, this is your opportunity for you see all the chemistry questions that have been set by the Join Admission and Matriculation Board for the 2025 examination.
You are specially welcomed to this website if you are visiting for the first time. This is a site where you can always have access to all the JAMB questions and answers for all the subjects even before you go into the examination hall.
In today’s article, I shall be discussing the JAMB chemistry questions and answers for 2025 examination. I have noticed that they have been a great search about the JAMB questions that have been taken on the first and second days of the examination by the most candidates who are yet to sit for the examination.
This is in a bid to get used to the kind of questions that Joint Admission and Matriculation Board have been asking since the start of the 2024 examination.
On this note, I will be taking you on the chemistry questions and answers for the year 2024. Most times, JAMB can repeat some of the questions that have been asked to candidates who took the examination on the previous days.
That is why it necessary that I publish this article here for the benefit of those who have not taken the examination yet. This article is for those who would like to know the exact questions that they are likely going to see on their own examination dates.
So it is advisable that you take every information contained in this article very serious if you really wish to have the benefit of not have written your examination on the first days.
JAMB Chemistry 2025
As mandatorily required by the Joint Admission and Matriculation Board for every year, all the candidates must write English language plus additional three subjects that are related to their chosen courses of study.
Chemistry is a science subject and optionally required subject for some courses and one of the compulsory subjects for most of the science related courses.
Some of the courses that require taking chemistry in JAMB examinations are as follows:
- Biological sciences
- Medical courses
- Agricultural sciences
- Physical sciences
- Engineering courses
- Some environmental science courses
- Some science-related education courses and so on.
Related Posts:
JAMB Government Questions And Answers 2025
JAMB Economics Questions And Answers 2025
How Many Questions are in JAMB Chemistry 2025
For those of you who have been try to know how many questions that are going to be contained in the 2025 JAMB Chemistry, I am going to provide answer to your questions in this section of the article.
In the previous years, that is, before the year 2017, the total number of questions that are expected to be answered by each candidate according to their respective subject combination is used to be 250.
For JAMB 2025, Chemistry will be 60 questions in number while all other subjects, including Chemistry will be 40 questions.
2025 JAMB Chemistry Questions for Day One
The following are the complete 2025 JAMB chemistry questions for the first day;
1. The movement of liquid molecules from the surface of the liquid to the gaseous phase above it is known as
A. Brownian movement
B. Condensation
C. Evaporation
D. Liquefaction
2. A mixture is different from a compound because
A. a mixture can be represented by a chemical formula while a compound cannot
B. the properties of a compound are those of its individual constituents while those of a mixture differ from its constituents
C. a mixture is always homogeneous while a compound is not
D. the constituents of a compound are chemically bound together while those of a mixture are not.
3. What is the percentage of sulphur in sulphur(IV) oxide
A. 50%
B. 66%
C. 25%
D. 40%
[S = 32 2, O=16]
4. A gas X diffuses twice as fast as gas Y. If the relative molecular mass of X is 32, calculate the relative molecular mass of Y.
A. 64
B. 128
C. 8
D. 16
5. 200cm3 of gas at 250C exerts a pressure of 700 mmHg. Calculate its pressure if its volume increases to 350cm3 at 75 °C.
A. 400.00mmHg
B. 342.53mmHg
C. 1430.54mmHg
D. 467.11mmHg
6. An element X has electron configuration 1s2 2s2 2p6 3s2 3p5. Which of the following statement is correct about the element?
A. It is a halogen.
B. It has a completely filled p-orbital.
C. It has 5 electrons in it outermost shell.
D. It belongs to group II on the periodic table
7. Beryllium and aluminum have similar properties because they
A. are positioned diagonally to each other
B. are both metals.
C. belong to the same group.
D. belong to the same period.
8. If the difference in electronegativity of elements P and Q is 3.0. The bond that will be formed between them is
A. ionic
B. metallic
C. covalent
D. co-ordinate
9. How many protons, neutrons and electrons respectively are present in the element 2760Co?
A. 60, 33, and 60
B. 27, 33 and 33,
C. 33, 27 and 27
D. 27, 33 and 27
10. The radioactive radiation used in studying the arrangement of particles in giant organic molecules is
A. β-particles
B γ-rays
C. α-particles
D. X-rays.
11. A silicon-containing ore has 92% 28Si, 5% 29Si and 3% 30Si. Calculate the relative atomic mass of the silicon
A. 28.00
B. 14.00
C. 29.00
D. 28.11
12. The nitrogen obtained from air has a density higher than the one from nitrogen-containing compounds because the one from air is contaminated with
A carbon (IV) oxide
B. water vapour
C. oxygen
D. rare gases
13. Water is said to be temporarily hard when it contains
A. CaSO4 and Ca(HCO3)2 salts
B. Ca(HCO3) and Mg(HCO2)2 salts
C. Ca(HCO3)2 and CaSO3 salts.
D. Mg(HCO3)2 and CaSO4 salts.
14. On exposure to the atmosphere, a hydrated salt loses its water of crystallization to become anhydrous. This phenomenon is referred to as
A. hydrolysis
B. efflorescence
C. deliquescence
D. hydgroscopy
15. 16. 55g of lead (II) trioxonitrate (V) was dissolved in 100g of distilled water at 20°C calculate the solubility of the solute in moldm³
A. 0.50g
B. 0.05g
C. 2.00g
D. 1.00g.
16. The dispersion of a liquid in a medium will give
A. an aerosol
B. an emulsion
C. a fog
D. a gel
17. The major and most effective way of controlling pollution is to
A. convert chemical wastes to harmless substances before releasing them into the environment.
B. improve machinery so that the substances released from combustion are less harmful
C. pass strict laws against it by individuals and companies
D. educate people on the causes and effects pollution.
18. The basicity of CH3COOH is
A. 3
B. 4
C. 1
D. 2
19. The colour of litmus in a neutral medium is
A. orange
B. purple
C. pink
D. yellow
20. The mathematical experience of pH is
A. log10 1/[OH–]
B. log10 1/[OH–]
C. log10 1/[H3O+]
D. log10 [H3O+]
21. Which of the following salts will turn litmus red?
A. Zinc chloride hydroxide
B. Sodium tetrahydroxonzincate(II)
C. Potassium hydrogentetraoxosulphate (VI)
D. Sodium trioxocarbonate(IV).
22. Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
In the reaction above, the oxidation number of the reducing agent changes from
A. +1 to +3
B. 0 to +4
C. 0 to +2
D. +1 to +2
23. H2O(g) + C(s) → H2(g) + CO(g)
The oxidizing agent in the reaction above is
A. H2(g)
B. CO(g)
C. C(s)
D. H2O(q)
24. Calculate the quantity of electricity in coulombs required to liberate 10g of copper from a copper compound
A 15196.5
B. 32395.5
C. 30156.3
D. 60784.5
[Cu=64, F = 96500Cmol-1]
25. How many faraday of electricity is required to produce 0.25 mole of copper? A. 0.50F
B. 1.00F
C. 0.01F
D. 0.05F
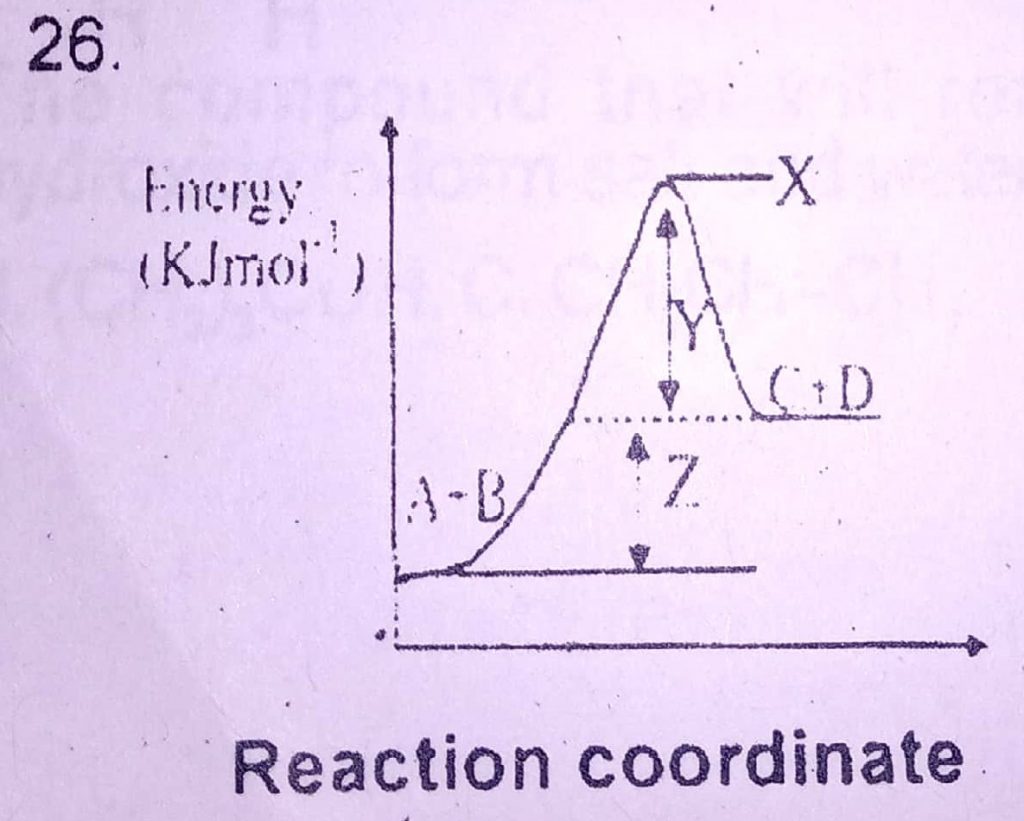
26. Z in the diagram above represents
A. entropy of reaction
B. heat of reaction
C. activation energy
D. free energy
27. If the change in free energy of a system is -899 Jmol-¹ and the entropy change is 10J mol-¹ K-1 at 25 °C, calculate the enthalpy change.
A. +649 Jmol-1
B. +2081 Jmol–¹
C. -2081 Jmol–¹
D. -649 Jmol–¹
28. In an equilibrium reaction, which of the following conditions indicates that maximum yield of the product will be obtained?
A. Equilibrium constant is less than zero
B. Equilibrium constant is very large.
C. ∆H-T∆S = 0
D. ∆H >T∆S
29. In a chemical reaction, the change in concentration of a reactant with time is
A. order of reaction
B. entropy of reaction
C. enthalpy of reaction
D. rate of reaction
30. Cr₂O²(aq) + H₂O: 12Cr₂0²–4(aq) + 2H+(aq).
What happens to the reaction above when the hydrogen ion concentration is increased?
A. The equilibrium position will shift to the left
B. More of the products will be formed
C. The reaction will not proceed
D. The equilibrium position will shift to the right.
31. Which of the following will liberate hydrogen from dilute tetraoxosulphate(VI) acid? A. Gold B. Lead C. Magnesium D. Copper.
Use the diagram below to answer questions 32 and 33.
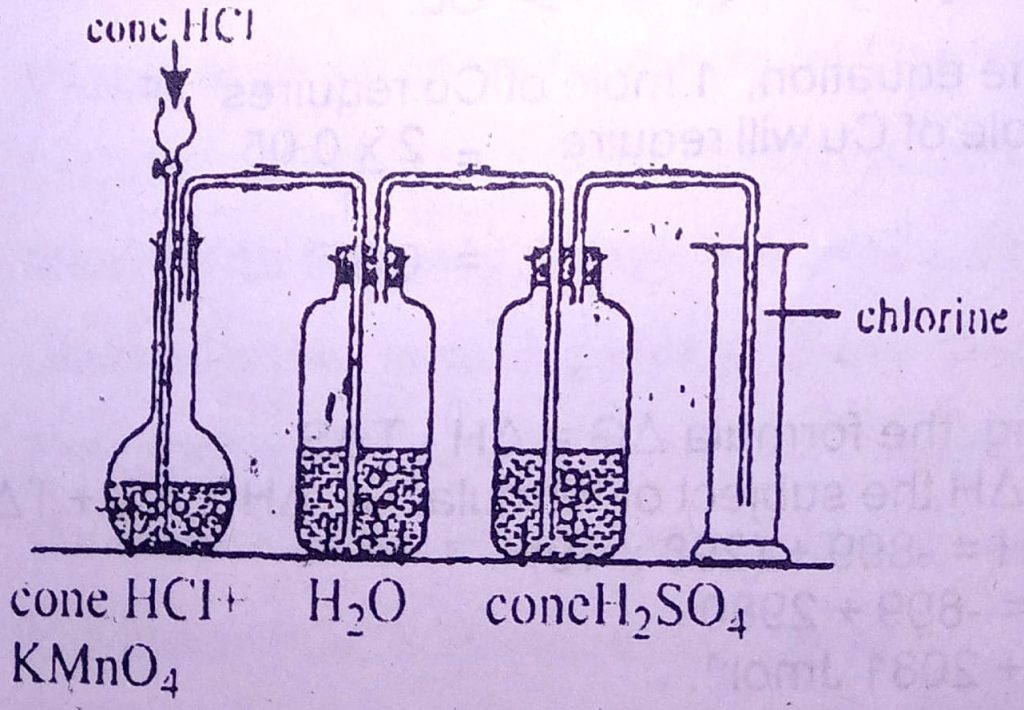
32. In the diagram, the function of the concentrated H2SO4 is to
A. remove odour
B. purify the gas
C. dry the gas
D. liquefy the gas
33. The gas that is remove by the water in the flask is
A. H₂
B. O₂
C. SO₂
D. HCI
34. Fluorine does not occur in the state in nature because
A. of its higher reactivity
B. it is a poisonous gas
C. it belongs to the halogen family
D. it is inert
35. In the extraction of sodium from fused Sodium chloride, the anode is made of platinum because
A. Chlorine does not react with platinum
B. Sodium is formed at the anode
C Chlorine is formed at the anode
D. Sodium does not react with platinum.
36. A compound that gives a brick-red colour to a non-luminous flame is likely to contain
A. aluminium ions
B. copper ions
C. sodium ions
D. calcium ions
37. In the electrolytic extraction of calcium from calcium chloride, the cathode is
A. iron
B. zinc
C. graphite
D. platinum
38. A few drops of NaOH solution was added to an unknown salt forming a white precipitate which is insoluble in excess solution. The cation likely present is
A. Al3-
B. Zn2+
C. Pb2+
D.Ca2+
39. The general formula of halolkanes where X represents the halide is
A. CnH2n+1 X
B. CnH2n+4X
C. CnH2nX
D. CnH2n+2X
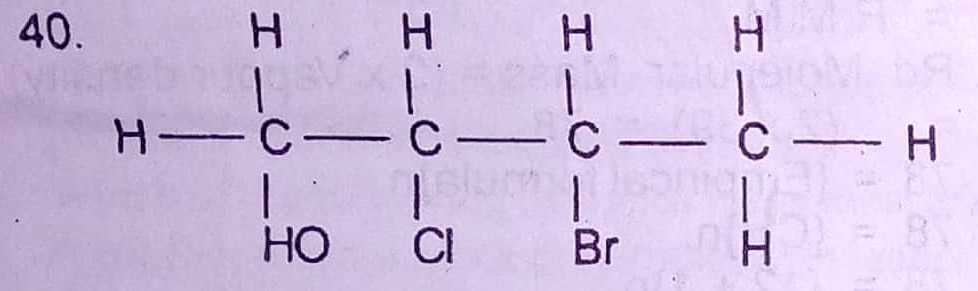
40. The IUPAC nomenclature of the compound above is
A. 2-chloro-3-bromobutanol
B. 2-bromo-3-chlorobutanol
C. 3-bromo-2-chlorobutanol
D. 3-chloro-2-bromobutanol
41. The alkanol obtained from the production of soap is
A. methanol
B. propanol
C. ethanol
D. glycerol
42. Ethyne is passed through a hot tube containing organo-nickel catalyst to produce
A. benzene
B. isoprene
C. polythene
D. ethanal
43. Due to the unstable nature of ethyne, it is stored by dissolving in
A. propanone
B. ethane-1, 2-diol
C. propanol
D. ethanoic acid
44. The process of converting starch to ethanol is
A. oxidation
B. cracking
C. distillation
D. fermentation
45. The polymer used in making car rear lights is
A. polyacrylonitrile polystyrene
B. perspex
C. bakelite
D. polystyrene
H+
46. CH3COOC2H5(l) +H2O(l) → CH3COOH(aq + C2H5OH(aq)
The purpose of H+ in the reaction above is to
A. decrease the rate of the reverse reaction
B. increase the yield of products
C. maintain the solution at a constant pH
D. increase the rate of hydrolysis.
47. A hydrocarbon has an empirical formula CH and a vapour density of 39. Determine its molecular formula
A. C6H6
B. C2H6
C.C3H8
D.C3H4
[C = 12 H=1]
48. Polystyrene is as packaging materials for fragile objects during transportation because of its
A. high compressibility
B. lightness
C. low density
D. high density
49. The process of converting linear alkanes to branched chain and cyclic hydrocarbons by heating in the presence of a catalyst to improve the quality of petrol is referred to as
A. blending
B. refining
C. cracking
D. reforming
50. The petroleum fraction that is used in heating furnaces in industries is
A. lubricating oil
B. diesel oil
C. gasoline
D. kerosene
See also:
JAMB Physics Questions and Answers 2025 for Day One
JAMB Biology Questions And Answers 2025
Answers to JAMB Chemistry 2025
- C
- D
- A
S = 32, O = 16
SO2 = (32 + (16×2)) = 64; % of S = 32/64 x 100/1 = 50%
4. B
5. D
6. A
7. A
8. C
9. D
10. D
11. C
12. D
13. B
14. B
15. A
Molar mass of Pb(NO3)2 = 331
16.55g of Pb(NO3)2 contains (16.55)/331 = 0.05mole
100g of water at 200C dissolves 0.05moles of Pb(NO3)2
Therefore, 1000g of water at 200C will dissolve 0.05×1000/100
= 0.5moldm-3
16. B
17. D
18. C
19. C
20. C
21. C
22. C
23. D
24. C
Cu2++ 2e– → Cu
2F → Cu
193000 → 64g of Cu
If 64g of Cu requires 193000C
Therefore, 10g of Cu will require 193000 x 10/64
= 30156.3C
25. A
Cu2++ 2e– → Cu
2F → Cu
From the reaction, 1 mole of Cu requires 2F
0.25 mole of Cu will require = 2 x 0.25/1 = 0.5F
26. B
27. B
Applying the formula, ∆G = ∆H – T∆S
Make ∆H the subject of the formula; ∆H = ∆G + T∆S
∆H = -899 + (298 x 10) = -899 + 2980
= +2081Jmol-1
28. B
29. D
30. A
31. C
32. C
33. D
34. A
35. A
36. D
37. A
38. D
39. B
40. A
41. D
42. A
43. B
44. D
45. B
46. B
47. A
Molecular formula = (Empirical formula)n
= R.M.M.
Rd. Molecular Mass = (2 x Vapour density)
= (2 x 39) = 78
78 = {Empirical formula}n
78 = (CH)n
78 = (12 + 1)n
78 = 13n
n = 78/13 = 6
Therefore, Molecular formula = (Empirical Formula)
= (CH)6
= C6H6
48. B
49. D
50. D
Reasons For Poor Performance in JAMB Chemistry
Many times, JAMB candidates have worried about their poor performance in chemistry even after they have taken enough time to prepare for their examination.
There are many reasons which can lead to scoring low in JAMB chemistry, which is what I am going to show you in this section of the article.
Reasons for poor performance in JAMB chemistry include the following:
- Inadequate Preparation
This is the most important aspect of the reasons for poor performance in JAMB Chemistry that should be considered.
It is obtainable in all areas that when there is poor preparation for any examination they will be poor result. As a good student, you are required to prepare for the JAMB chemistry examination to the point that you would be sure of scoring high.
2. Poor Time Management
Time management is another important factor that can lead to poor performance in every examination. You should learn how to manage your time especially when you are in the examination hall.
The practice of time management starts from the period of the candidate’s preparation for the examination. Once you can answer your past questions comfortably with the limited time that will be provided, you will still manage your time very well on the examination date.
3. Lack of Adherence to Given Instructions
There are some students who are fond of ignoring instructions when come for examination. Jumping into answering of questions without reading the given instructions is very wrong. This contributes in a big way to poor performance in the examination.
If you want to have good performance in JAMB chemistry, you have to strictly pay close attention to any given instructions.
4. Submitting Incomplete Answers
When a candidate submits the examination without attempting all the questions, it will automatically affect his/her score. It is important you answer all the questions before your submission.
If your submission is by mistake, make sure that you use key R to reverse it and complete your examination.
What You Should Read for JAMB Chemistry 2025
In JAMB chemistry examination, they are some topics that are considered very important. These topics are where most of the questions that are repeatedly asked by the JAMB for every year.
As you continue to read this section, I am going to reveal those topics to you. If you are able to read the chemistry topics that I am going to show you here very well before the examination, it means that you can attempt up to 90% of JAMB chemistry questions correctly.
These topics include the following:
- Nature of Matter
- Separation Techniques
- Atomic Structure
- Chemical Combination
- Kinetic Theory of Matter
- Gas Laws
- Acids, Bases and Salts
- Periodic Table
- Non-metals and their Compounds
- Metals and their Compounds
- Electrolysis
- Energy and Chemical Reactions
- Chemical Equilibrium
- Rate of Reaction
- Air and Air Pollution
- Water, Solution and Solubility
- Shapes of Molecules and Solids
- Radioactivity
- Hydrocarbon
- Organic chemistry
However, you should note that reading with the JAMB syllables is the best practice. The topics listed above are just suggestion of areas that you can concentrate so as to get a good result in the JAMB chemistry examination.
How to Answer JAMB Chemistry Questions
For every examination, there are/is principle(s) that must be followed by the candidates to ensure that the required level of success in the examination is achieved at the end.
In this section, I am going to reveal to you those principles that are applicable to JAMB chemistry examination for an excellent performance.
First of all, I would like to let you know that it is possible to for a candidate to score 100% in JAMB chemistry. That is, if such candidate follows the due rules and regulations required for answering the questions.
For those that would like to score above 300 in their UTME, it is advisable that you take the information contained in this section very serious.
The basic principles for answering JAMB chemistry questions are as follow:
- Read Instructions
Once you login into the system and you have come to the chemistry section of the CBT, the next thing that you must do first is to read through the instructions on top of the page before you can proceed with reading the questions.
Most times, some instructions are specific to some questions, maybe one or two questions. Here, you still have to take those instructions very serious.
2. Read Carefully
Before you can proceed to answering JAMB chemistry questions, it is highly recommended that you read carefully to understand any question before you can select answer for it.
It is possible to see questions that would be similar to what you have been seeing before, probably in past questions, but they are not the same.
This is the point where it pertinent that you meticulously go through the question before you answer, to avoid choosing the wrong option.
3. Start with the Simplest
The complexity of every question in JAMB chemistry varies. After you have gone through the questions, you would be able to tell which of the questions are simple and the ones that are difficult.
The best approach in such case requires that you answer the questions from the simplest to the most complex ones. This is important because it will help to cushion you against examination tension.
Not only that, it also helps in the management of your time. If you are finding any question difficult to answer, you have to leave it and go to the next. Thereafter, you can re-visit those questions that you have left unanswered.
4. Attempt all the Question
Though it is advisable that you start answering your question from the simplest, leaving the more difficult ones at the first attempts, it does not imply that you should submit you examination without answering those ones you skipped.
It quite understandable that you may not know all the asked questions, you are still required choose your answers even the ones that you do not really know very well.
Sometime, your guess can fall in place and become the right answer. So always ensure that you attempt all you question before you submit.
5. Review your Answers
After you have attempted the entire given questions, you still have to go through all the 40 questions to check for any errors and possible corrections.
By going through the questions, you would be able to see any skipped question(s), if any and the ones you mistakenly clicked the wrong options.
6. Master your Shortcut Keys
As far as JAMB Computer Based Test is concerned, a good knowledge of the computer shortcut key is very important. Most times, the shortcut keys are given in the instruction section of the examination.
You have to get used to them so as to avoid using the wrong keys that will disrupt your examination. For instance, you might not mean to submit you examination but due to the lack of good understanding of the shortcut keys, you will mistakenly submit without completing your examination.
Also, there are times when your computer mouse may not be responding as expected. If you understand the keys very well, you will not have to worry yourself much. All you have to do is to switch from mouse click to using keys.
Some of the JAMB CBT keyboard guides are;
- A – for option A
- B – for option B
- C – for option C
- D – for option D
- N – for next question
- P – for previous question
- S – for save and submit
- R – for reverse, in case you saved by mistake without finishing you examination
You May Also Like:
Recommended Links
Complete 2025 WAEC Physics Questions and Answers | Objective & Theory
Complete 2025 WAEC Biology Questions and Answers | Objectives and Theory
Tips on How to Pass WAEC at One Sitting
2025 WAEC Biology Practical Specimen | Questions And Answers
Updated May/June WAEC timetable for 2025 | Nigeria, Ghana, Gambia, Sierra Leone & Liberia
I hope that you found this article very helpful. For any questions about JAMB Chemistry Questions and Answers for 2025, kindly make use of the comment section below.
Please do well to share this information to your colleagues via any of the available social media platforms.


Pls,do you have online classes or videos for practice?
You shall be notified once it is ready